As the only currently approved treatment option for AATD lung disease, plasma donor-derived Alpha-1 Antitrypsin protein (human alpha-1 proteinase inhibitor [A1PI] augmentation therapy) remains the current standard of care. pdAAT has improved patient outcomes but an opportunity exists to advance the standard of care in AATD.
At Inhibrx, a group of passionate scientists came together with a mission to develop a novel therapy for patients living with AATD. Using science and innovation, the journey to develop the investigational drug INBRX-101 began.
INBRX-101 is a recombinant (artificial) form of augmentation therapy that has been engineered to last longer in the body. This means that INBRX-101 could potentially be given less frequently than currently approved pdAAT augmentation therapies, like Zemaira®, while keeping the levels of AAT in the normal range.
The first leg of the journey: The Phase 1 study
INBRX-101 was studied in an investigational study in 31 adults with AATD. This Phase 1 study looked at the safety, pharmacokinetics (levels of drug in the body), and pharmacodynamics (how the study drug affects AAT levels) of INBRX-101. In the completed Phase 1 study, INBRX-101, when administered at single doses and multiple doses up to 120 mg/kg administered every 3 weeks for up to 3 doses, was found to be safe and tolerable.
Overall, the side effects reported were generally mild or moderate in severity and resolved on their own without needing additional treatment. The most frequently reported side effects were fatigue and infusion-related side effects (e.g., itching, blood pressure increase, etc.). This study also showed that the levels of functional AAT (the protein that helps protect the lungs from damage) that were measured in the blood were similar to levels of AAT that are seen in people without AATD.1

The trail gets steeper as the journey continues
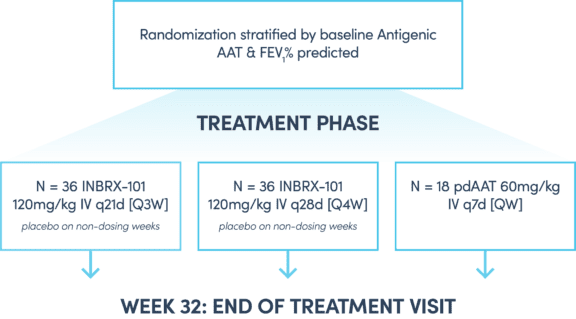
INBRX-101 is now being investigated in ElevAATe, a phase 2 randomized, double-blind, multicenter, active-control study designed to assess INBRX-101 compared to Zemaira® in patients with AATD-related emphysema.
This investigational study will evaluate the safety and therapeutic effects of two dosing regimens of INBRX-101 (a recombinant [artificial] form of alpha-1 proteinase inhibitor [A1PI] augmentation therapy) compared to Zemaira® (a human plasma derived A1PI augmentation therapy) in patients with AATD emphysema. Current approved A1PI augmentation therapies, like Zemaira®, replace the AAT protein in patients who are deficient. These A1PI augmentation therapies require long-term treatment administered via weekly infusions to raise AAT levels. INBRX-101, as a recombinant A1PI augmentation therapy has been engineered to last longer in the body. This means that INBRX-101 could potentially be given less frequently than currently approved A1PI augmentation therapies, like Zemaira®.
The ElevAATe study is now enrolling.

The journey doesn’t end with ElevAATe
For participants who complete the 32-treatment portion of the ElevAATe study there is the opportunity to receive INBRX-101 in an open-label study evaluating the effect of INBRX-101 on long-term safety and efficacy (ElevAATe-OLE).
In this study, participants will receive INBRX-101 once every 3 weeks for approximately 3 years. The study drug and study visit assessments will be provided at no cost to participants and this study is planning to include home/remote healthcare options to potentially allow some participants to make less frequent visits to the study site.
How do I enroll?
To join the journey and learn more about the ElevAATe and ElevAATe-OLE studies, complete the following pre-screening form to see if you may qualify. Please note this is not a full list of criteria and additional screening may be needed. Please click the link below to begin.
To access the Alpha-1 Foundation Pre-Screening, click here.
For any questions or concerns, please contact the Alpha-1 Foundation Research Registry Coordinators at 1-877-228-7321 ext. 245 or 252, or alpha-1registry@alpha1.org.
Kuhn BT, Veale A, Farah H, et al. Recombinant human AAT protein INBRX-101 demonstrates potential to achieve lung penetration and normal functional serum AAT levels in patients with AAT deficiency. Presented at: the ATS International Conference 2023; May 19-24, 2023; Washington, DC.
INBRX-101 is an investigational treatment for which we have not yet obtained marketing authorization, and its safety and efficacy have not been definitively established.











