Exciting breakthrough in Alpha-1 research by Beam Therapeutics shows success in restoring the mutated Alpha-1 gene to normal. This correction has the potential to halt liver and lung damage in patients with Alpha-1 Antitrypsin Deficiency. A1F is excited to share this news with Alpha-1 community that was published in the New York Times on March 10, 2025.
“The positive initial data for Beam-302 represents an innovative medical breakthrough that gives patients suffering from Alpha-1 Antitrypsin Deficiency hope and promise that a novel an and effective treatment is on the horizon.” Scott Santarella, CEO Alpha-1 Foundation (A1F)
“Since Ron Crystal’s 1987 publication demonstrating that delivering a copy of the human alpha-1 gene to animals could result in production of the normal protein, we have been dreaming of gene therapy as a treatment for this disease. These results suggest that correcting the mutation responsible for the disease can restore gene function in patients with AATD, an exciting step towards a genetic and potentially curative therapy.” Dr. Andrew Wilson, A1F Scientific Director
Kolata, Gina “Mutated DNA Restored to Normal in Gene Therapy Advance”
The New York Times, March 10, 2025
Researchers have corrected a disease-causing gene mutation with a single infusion carrying a treatment that precisely targeted the errant gene.
This was the first time a mutated gene has been restored to normal.
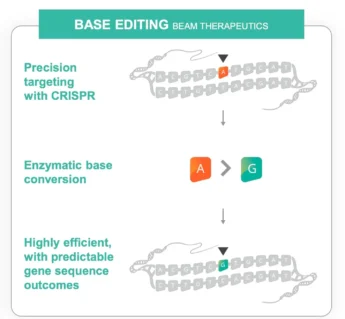
The small study of nine patients announced Monday by the company Beam Therapeutics of Cambridge, Mass., involved fixing a spelling error involving the four base sequences — G, A, C and T — in DNA. The effect was to change an incorrect DNA letter to the right one. The result was a normal gene that functioned as it should, potentially halting liver and lung damage of patients with a rare disorder.
“This is the beginning of a new era of medicine,” said Dr. Kiran Musunuru, a gene therapy researcher at the University of Pennsylvania’s Perelman School of Medicine.
He added that the method offers the hope of treating other genetic diseases precisely by fixing mutations — an alternative to current gene therapies, which either add new genes to compensate for mutated ones, or slicing DNA to silence genes.
Dr. Musunuru is a co-founder and equity holder of Verve Therapeutics, a gene therapy company, and receives funding from Beam Therapeutics for research, but not for this study.
Dr. Richard P. Lifton, president of Rockefeller University and head of its Laboratory of Human Genetics and Genomics, said the sort of gene editing Beam did, rewriting genes with an infusion, “is a holy grail” that “has the promise for being a one-and-done kind of therapy.”
Dr. Lifton is a director of Roche Pharmaceuticals and its subsidiary Genentech.
Despite the study’s small size, he said the results are “a very impressive advance and very promising.”
The study involved patients who have alpha-1 antitrypsin deficiency, or AATD, a genetic disease that affects an estimated 100,000 Americans, mostly of European ancestry. That makes it as common as sickle cell in this country. It is progressive and incurable.
The alpha-1 antitrypsin protein is made in the liver and normally goes to the lungs and protects them from inflammation from inhaled irritants like smoke or dust. But in people with the disease, a single change in a DNA letter in the gene results in a misshapen and nonfunctional protein. The result is often emphysema or chronic obstructive pulmonary disease in unprotected lungs.
But many of the aberrant alpha-1 antitrypsin proteins never get to the lungs and instead build up in patients’ livers, often causing cirrhosis.
The gene editing was simple for patients. They sat in a chair for a couple of hours while lipid nanoparticles, like those used in Covid vaccines, were infused into their blood. The nanoparticles did not hold vaccines, though. Instead, they encased a microscopic gene editor. The lipid casing protected the editor on a journey to the liver.
When the nanoparticles reached the liver, the lipid layer peeled off, releasing the editor — a disabled CRISPR molecule that acted like a GPS for the genome and an enzyme to fix the mutation. The CRISPR molecule crawled along the patient’s DNA until it found the one incorrect letter that needed to be repaired among the three billion DNA letters in the genome. Then the editing enzyme replaced that letter with the correct one.
The study divided the patients into three groups and tested three different doses of the gene editor. Those who got the highest dose made enough normal alpha-1 antitrypsin to be in a range where no more damage should occur. There were no serious side effects, said John Evans, Beam’s chief executive officer.
Beam will now be offering the higher dose to the patients who got the lower doses in the company’s study. Beam will also study the treatment in more patients, and test an even higher dose of its gene editor.
“And then we immediately have to think about how we can get this approved,” Mr. Evans said.
Dr. Noel McElvaney, a professor at the Royal College of Surgeons in Ireland and an investigator in the Beam study, said there’s a real need for an effective treatment to halt the damage done by the mutated gene. He said he sees patients in their 30s and 40s with severe emphysema and “really bad liver disease.” And, he said, “by the time we see them there is already a significant amount of damage.”
For those suffering the worst effects of AATD, he said, the new gene therapy is “a major major breakthrough.”
“The big pro” of the new treatment, he said, is that “it theoretically cures the liver and lung disease in one go.”
Dr. McElvaney added, though, that “like all genetic interventions, we have to follow up for a long time to make sure it’s as good as we think it is.”
But patients now have renewed hope, said Dr. Andrew Wilson, scientific director of the Alpha-1 Foundation, an advocacy group.
“We have been dreaming of gene therapy as a treatment for this disease,” he said.
Read the original article at The New York Times website here.













I have been diagnosed with Alpha 1 .I’m ZZ. My father is 99 and is Alpha ZZ. My sister is ZZ and my son has ZZ. We need this to stop! I have cirrhosis and emphysema. I’m waiting now to see if my insurance will pay for infusions. They haven’t gotten back with me or my Doctor. I’m praying this new treatment can be rushed through for all of us. The Mc Cormick gene is not something any of my family wants! God speed in getting this approved!
How can us Alpha ZZ patients get to this study? I’ve had this for 5 years almost 6 and I’m 35 it’s been very very hard on me. I’d love to be that risk willing taker if it could help not only myself but a lot of others! Please more info and how to be in this?
Has the process begun to get this treatment approved? And when do you think it will be approved? I have been diagnosed with Alpha 1 but show no symptoms. My brother just went through having a liver transplant as a result of his diagnosis. So I am very curious and interested in being part of this new “cure”. How can I be a part of the Beam-302 project? Thanks
Chris
Im so happy this is even in the works. I hope i get to see it in my lifetime. It already took my grandmother and nother. But very curious if it’s for all alphas. Im a rare allele. I assume there will be a study we can read one day soon?
I’m an SZ. I’m assuming this was only tested on ZZ for now — in theory, I’d expect this to turn me into an MS, which would be a game changer, unless they made versions for both Z and S. I’ll be curious to follow this.
I’m a MZ starting to have liver and lung issues, my 3 daughters are MZ also, I hope and pray this treatment is approved as soon as possible to save lives affected by this gene disorder. I hope to be part of the trials, when do they start in Missouri?
For me and my family members affected this could literally save our quality of life – hoping and praying it is available to us in time. Could help so many deserving people.
I pray this is the answer we’re waiting for. Also, I hope MZ are treated the same as ZZ. There are many of us that are desperately ill.
Wow! So exciting, and so meaningful for those of us with Alpha 1. Looking forward to the future of my kids and grandkids not having to suffer! Thank you, SCIENCE and BEAM!!!!
My baby is zz praying that this becomes available for him.
My daughter and I are both MZ. I have liver and lung involvement and she has started having liver involvement. I would love to be considered for this research program.
I have the alpha 1 zz type. And i so wish for this to be available in sweden in a very near future. My liver is ok for now tho ive got fatliver but that i change with a better aproach on what i eat and exercise, also have emphysema on my lungs.
i have the the Alpha1
In tears over the hope for so many, thank you for the research.
Crying over hear as well. A fellow ZZ. I never thought I would see the day. Stay in hope for here it is
I would want to be in trial. Was told I have a rare mutation.
I was diagnosed 2 and a half years ago and and although I’ve had the prolastin treatment it seems to be stairstepping downward usually degrading during the winter .months. Hopefully something can produce a better result.
Really hope this works out, curious as to results from this vs treatment with enzyme replacement beyond the fact this is supposed to be one and done as opposed to weekly treatment
Amazing and great breakthrough for AAT patients and the genetic disease treatment community. I worked on AAT twice in my career: once as a postdoc doing clinical and protein structure studies on AAT and again at Genzyme when the company attempted to produce AAT in goat milk to supply as an infused product. With AAT deficiency affecting a lot of people, even young children, it’s great to see the progress!
Excellent to see this work. Yes, there is need for further safety trials no doubt, and it will take brave and risk willing volunteers to join such trials.
I would so hope to be on the list of receivers. Already having Bronchieacasis and NTM I would like to know if it would put a better result to the complications of having AlphaZZ.
I live in Puerto Rico and this sept had a baby boy which had high bilirubin leves and liver enzymes got a liver biopsy at 2 months and showed fibrosis and genetics 🧬 testing confirmed serafin1 .. really hoping to see this new medication 💊 in my baby boys life.
What is the likely time frame for this treatment to be approved and made available to people suffering with AATD?
It may come too late for a friend of mine I fear!
How can one get into the study?
Amazing and wonderful! To think of all the people and families that can be helped!!! More birthdays, anniversaries, loving moments, and joys to be had boy affected individuals and their families, and loved ones!
Can this also be done for proline deficiency?
Thank you science and research!!
Lord have mercy, this makes me cry.
What an absolute miracle if this gets approved.
Won’t save my mom, but it sure will help others.
Wonder if carriers would need to be “fixed”?
Thank you Beam!
I have suffered from this disease my entire; I am now 80.I hope this incredible achievement will help everyone, especially the young people. I WANT TO KNOW MORE AS THIS GENE INNOVATION PROGRESS. THANK YOU SO MUCH FOR YOUR SUCCESS.
I am a zz alpha 1. I just started infusions. This is the best news ever for all alphas but also so many other genetic diseases. Thank you for caring. It means the world to this alpha.
Hi there, I’m a ZZ here in Australia too. They apparently just started the first trial last Friday I believe.. at Chermside Brisbane ☺️
I have been diagnosed for years. My breathing is getting worse. I would like to participate in the beam-302 study.
Thank you so much when can clinical trials start in Australia.
I am interested as I have alpha 1 I’m zz with severe emphysema I will follow on alpha 1 foundation site